FDA CLEARANCE GRANTED TO WISHBONE MEDICAL’S PEDIATRIC PRIMA FEMORAL LOCKING PLATE SYSTEM
WishBone Medical, Inc., a leader in pediatric orthopedic medical devices, today announced it has received U.S. Food and Drug Administration (FDA) 510(k) clearance on the PRIMATM Femoral Locking Plate System, which comprises proximal and distal plating options designed for pediatric patients, along with single-use, sterile packed instrument kits for streamlined OR efficiencies.
The PRIMA Femoral Locking Plate System helps surgeons better address proximal and distal deformities and trauma applications of the femur in pediatric patients. Designed to be anatomical, stable, and efficient, the system offers bendable templates to simplify plate selection as well as a wire guide to assist with osteotomies and plate positioning during surgery. The smaller, low-profile plates will be available in two sizes (3.5mm & 4.5mm).
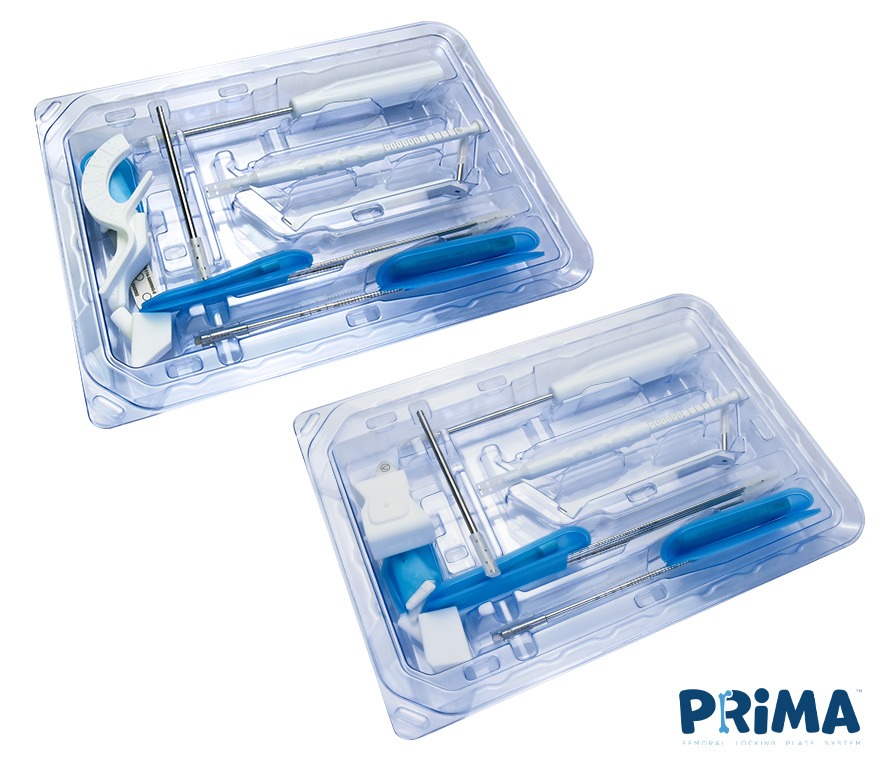
Delivering the benefits of single-use, sterile packed kits
- All instruments within the PRIMA Femoral Locking Plate System are new & pristine in sterile packed kits, reducing the risk of infections.
- Eliminates the need for metal trays, thereby eliminating washing, sterilization and decontamination time and cost.
- Streamlined component list. Only 13%-22% of instruments within traditional metal reusable trays are estimated to be used during the entirety of a procedure. (1)
- Potential reduction in surgical delays or cancellations due to non-sterile, non-functioning, or missing instruments.
- Operating room costs are $36-$37 per minute: delays related to missing or faulty instrumentation may increase costs by up to $260 per case. (1)
1. Apurva Shah, MD, MBA. The Value Proposition of Single-Use Sterile Procedure Kits. May 2021


